Introduction
This release marks yet another major update of the crmPack package: Backfill cohort simulations are now supported in the Design class, powered by the new Backfill class. This also leads to breaking changes in the Data and GeneralSimulationsSummary classes; both classes gain new slots.
Note: Please regenerate any existing Data or Design objects as well as resulting SimulationsSummary objects; serialized objects from previous versions will not be compatible.
Backfill cohorts
Backfilling cohorts are increasingly used in dose escalation studies, see Barnett et al. (2023). The idea is that once a dose level has been tested and found to be safe, then while the dose escalation continues at higher dose levels, additional patients can be enrolled at the lower dose levels to gather more data on safety and/or efficacy. This is particularly useful in trials where patient recruitment is slow or when there is a need to gather more information on lower dose levels for regulatory or clinical reasons.
Sometimes health authorities are asking to include the backfilling cohorts in the simulations to check the operating characteristics (especially PMDA). In addition, it is useful to get more precise operating characteristics for the overall trial by actually simulating the backfilling cohorts, when they are part of the actual clinical trial design.
Hence, with crmPack version 2.1 and higher it is now possible to include backfilling cohorts in the simulations. And don’t worry that it might be complicated: The detailed vignette will show you exactly how to do this.
Example
Let’s look at a concrete trial example:
We define a random number of patients for each backfill cohort, with a minimum of 1 patient and a maximum of 6 patients. Backfill cohorts can only be opened once at least 3 dose escalation cohorts have been completed. Note that this will lead to a delayed opening of cohorts 1, 2, and 3 for simultaneous backfilling. In addition, at least one response must have been observed at the cohort’s dose level or a lower dose level before it could be opened for backfill. For example, if there is no response observed in cohort 1 at the lowest dose level, then it will not be opened for backfilling at all. We will be able to specify the assumed dose-response probability function used in the simulations. Recruitment into backfill cohorts is slower than dose escalation cohorts, with a ratio of 1 backfill patient for every 2 dose escalation patients. When multiple backfill cohorts are open, then the highest dose level is recruited into first. The total maximum number of backfill patients is set to 20.
Sounds complicated, right? You probably don’t want to code this from scratch. Here is where crmPack comes in and makes it super easy to simulate this and other simpler or complex backfilling scenarios.
Here is how a concrete simulated trial might look:
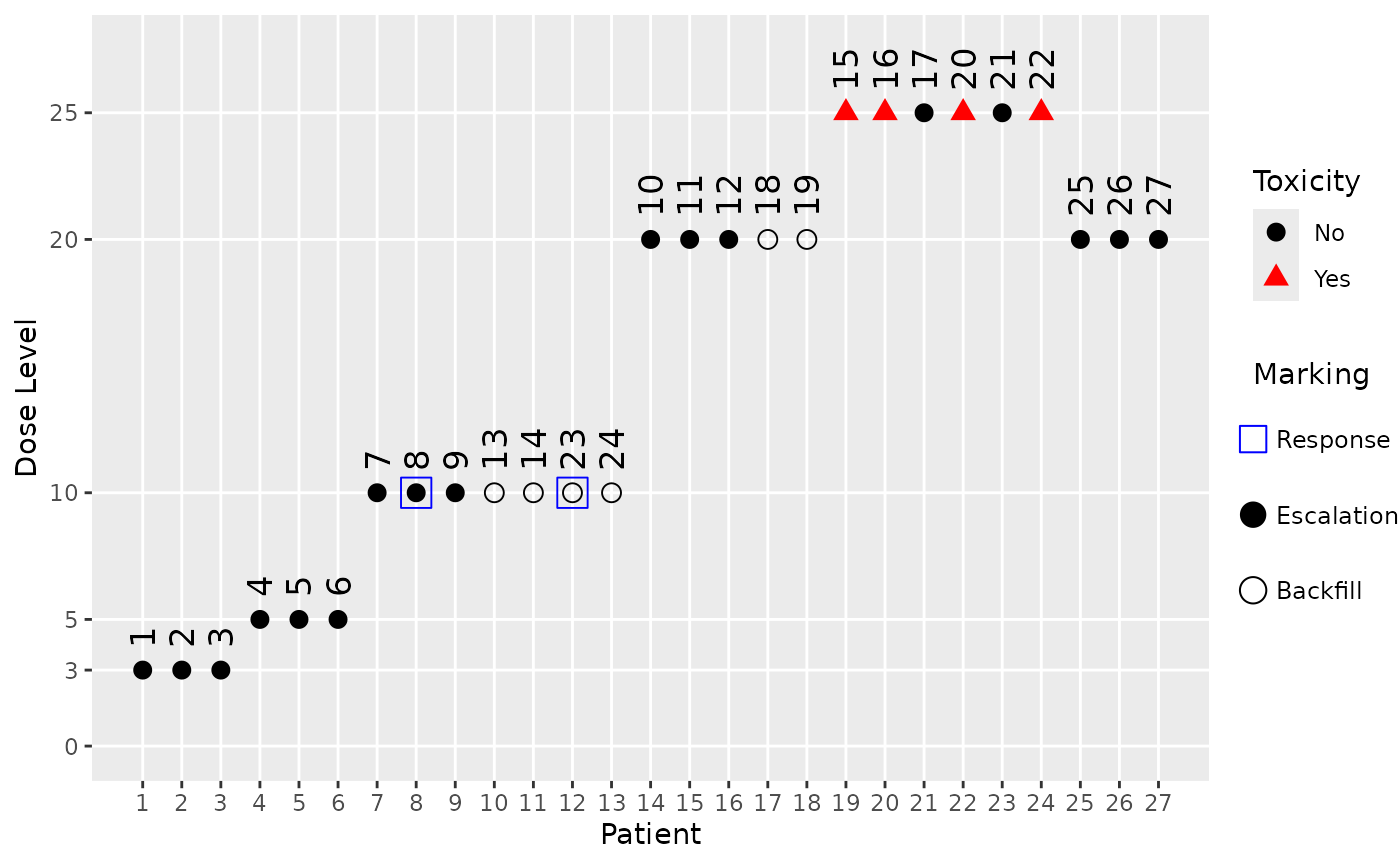
Here we can see that the starting dose cohort at dose 3 as well as the next one at dose 5 did not produce any responses, hence no backfill cohorts could be opened at these dose levels. Only after dose escalation reached dose 10 in cohort 3 and one response was observed there, a backfill cohort could be started there (producing yet another response) and at the higher dose 20. This is because we assumed a monotone dose-response relationship and included lower doses in our response assessment when defining the backfill cohort opening rule.
Conclusion
With backfill cohort simulation, crmPack offers yet another important feature for supporting the implementation of modern model-based dose escalation trial designs.
We invite interested pharmaceutical companies and CROs to join this collaborative effort to secure the future of crmPack and advance the field of adaptive dose escalation trial designs - please reach out to if you are interested in a first informal chat!