library(crmPack)
myData <- Data(
x = c(1, 1, 1, 3, 3, 3, 6, 6, 6),
y = c(0, 0, 0, 0, 0, 0, 0, 1, 0),
doseGrid = c(
1, 3, 6, 12, 24
)
)
plot(myData, blind = TRUE) + ylab("Dose")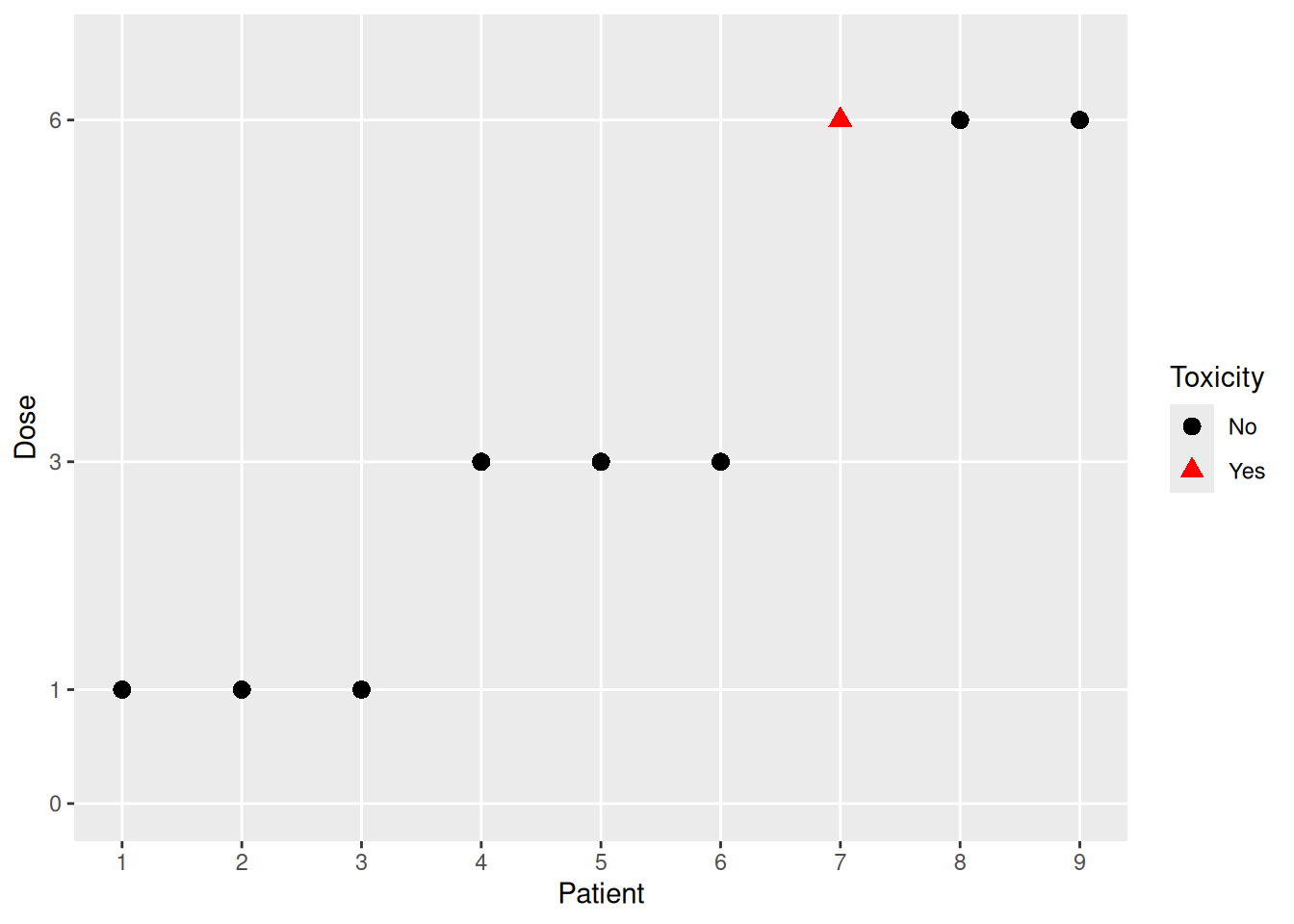
Daniel
December 1, 2025
Adaptive dose escalation trials are a key tool for Phase 1 development both in oncology and non-oncology settings. The R package crmPack has been developed over the last 11 years to facilitate the design and analysis of these trials. crmPack implements a wide range of dose escalation designs, ranging from classical and modern continual reassessment methods (CRMs), based on dose-limiting toxicity endpoints and dual-endpoint designs (e.g., plus biomarker or clinical endpoints), and permitting Bayesian and non-Bayesian inference (Sabanés Bové et al. 2019).
On 29th November 2025, a major milestone was reached. crmPack version 2.0 was released to CRAN, featuring a complete refactoring of the code base, extensive new functionality, comprehensive tests, and improved documentation - please have a look at the release announcement for details. This release marks the culmination of years of collaborative development by a team of statisticians from multiple pharmaceutical companies and academic institutions. We had previously the joy of sharing a bit of the story of the collaborative crmPack development at the ISCB 2022 conference (Boix and Günhan 2022), and we are going to describe this again here to celebrate the team’s success!
We also describe the innovative funding model to ensure the sustainability, documentation and training of crmPack and to develop new functionalities, which has the potential to make crmPack the new standard for adaptive dose escalation trials. The model is along the lines of the rpact funding model (see RPACT SLA), which has been successful for over 8 years now.
First, we want to acknowledge all the contributors who have made crmPack what it is today:
From Bayer: Clara Beck, Oliver Boix, Prerana Chandratre, Robert Adams, Dimitris Kontos (ClinBAY/Bayer)
From Genentech: Jiawen Zhu, Ziwei Liao
From Roche: John Kirkpatrick (now Astellas), Giuseppe Palermo, Guanya Peng, Doug Kelkhoff, Wojciech Wojciak (now independent), Uli Beyer
From Merck: Marlene Schulte-Goebel, Burak Kuersad Guenhan
From Academia: Wai Yin Yeung (University of Lancaster, now Roche), Thomas Jaki (University of Lancaster/Cambridge/Regensburg)
Adaptive dose escalation trials play a crucial role in the early stages of drug development. They aim to determine the appropriate dosage for a new drug in humans by gradually increasing the dosage in a stepwise manner. These trials gather data on both efficacy and safety of the drug, balancing therapeutic effect with the avoidance of excessive toxicity.
Conducted with a small overall sample size (typically 20-50 patients), these trials allow close monitoring and analysis of the drug’s performance. They are applied across various therapeutic areas and are not restricted to oncology.
Common design approaches include:
crmPack is focused on model-based designs, but also includes rule-based designs for comparison. We also plan to add model-assisted designs in future releases.
We are using crmPack here to illustrate a typical dose escalation trial’s course:

We see that 3 patients each were treated sequentially at the dose levels 1, 3, and 6, with one dose-limiting toxicity (DLT) observed at dose level 6. Dose escalation designs answer the question of which dose to recommend for the next cohort of patients based on the observed data, and how to estimate the maximum tolerated dose (MTD) at the end of the trial.
If you’d like to try crmPack to do so, please check out our introductory example!
The crmPack package is a specialized R package for dose escalation trials. Its initial CRAN release was in 2016, making it widely accessible to the R community. The package offers higher flexibility compared to other software, thanks to its modular design principles using S4 classes.
Key features include:
crmPack provides a highly flexible framework for the design and analysis of dose escalation trials. The framework’s key components include data handling, model specification, design rules, and stopping criteria, all working together to provide comprehensive trial design capabilities.
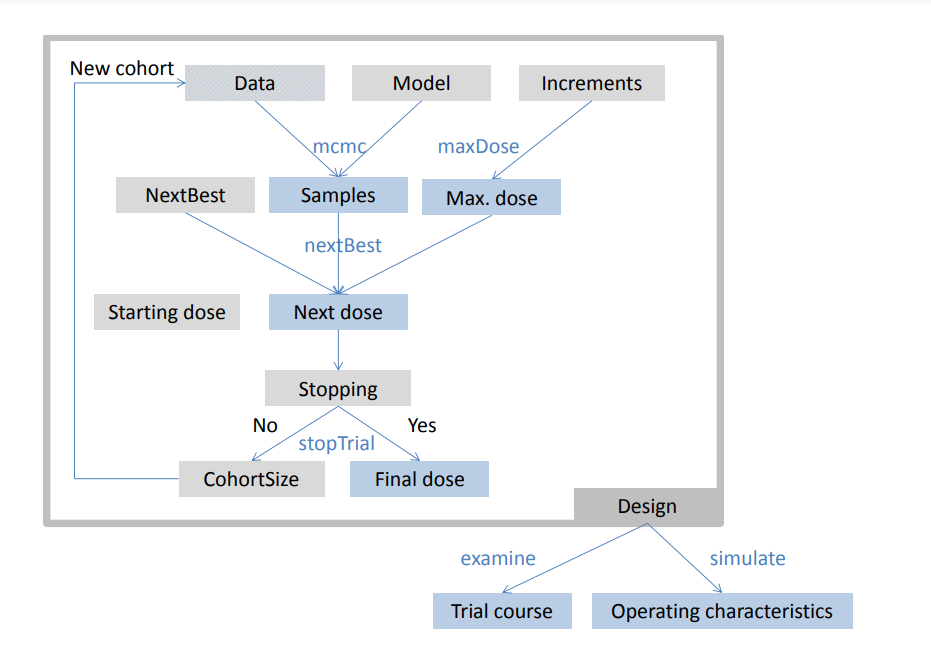
The development of crmPack has been a collaborative effort spanning over a decade:
Several alternative options exist for implementing dose escalation trial design:
Proprietary software: FACTS, East Horizon
Websites: TrialDesign.org, MD Anderson Biostatistics Software
Open source R packages:
bcrm, blrm, dfcrm, OncoBayes2BOINescalationcrmPack stands out by:
The following chart shows the median daily downloads of crmPack from CRAN each month since 2016:
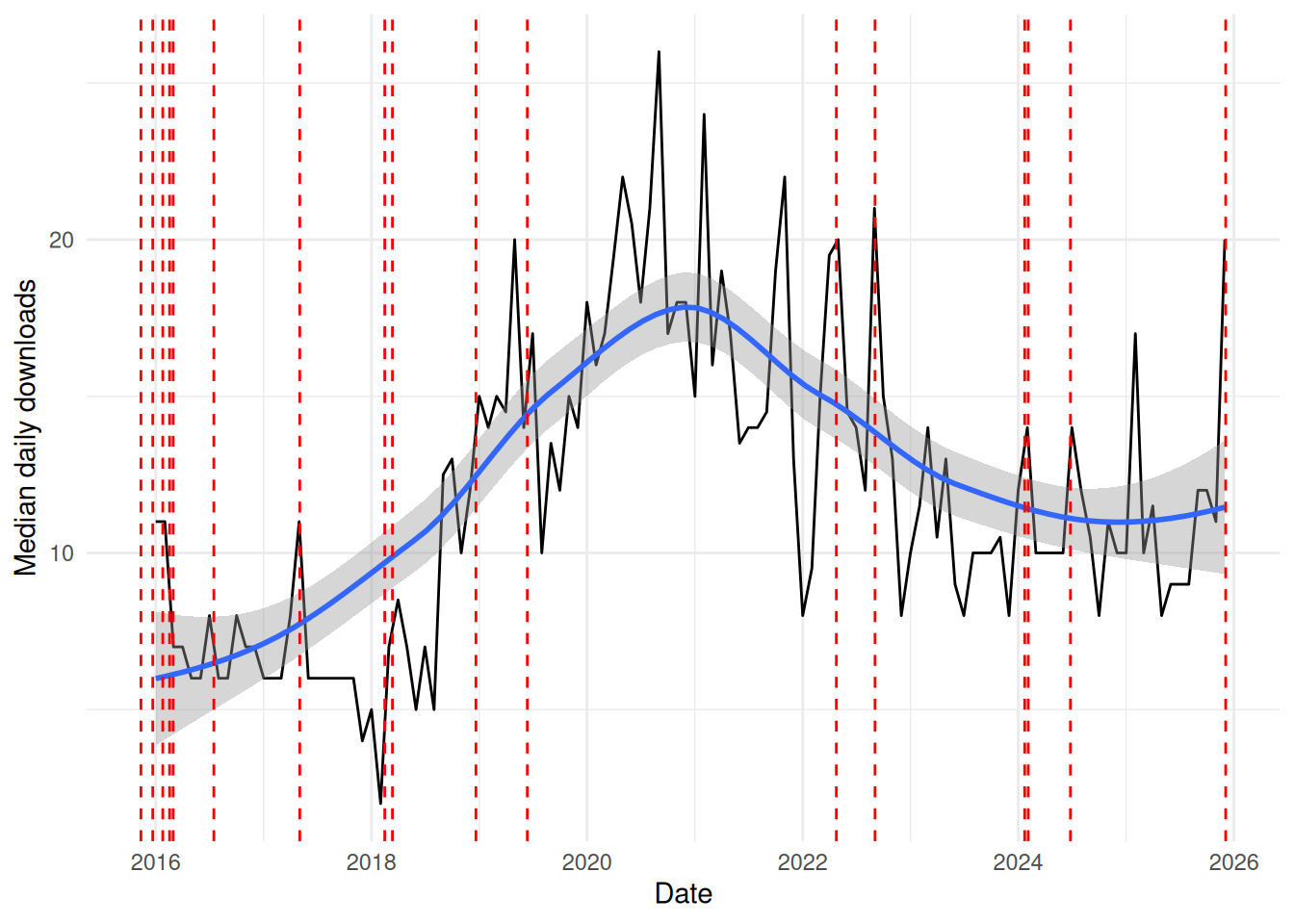
crmPack from CRAN (obtained via cranlogs)We aim to increase the visibility and usage of crmPack further through improved documentation, training, and support - as well as adding significant new features through the collaborative funding model.
The impact of crmPack is best illustrated through feedback from its users:
Burak and Marlene (Merck):
“We already used and continuously use successfully for clinical trials in regulatory context, and we appreciate the flexibility that you can adapt it to your needs. As we were part of development team, we are aware that development version has a high software quality. We have very positive experience collaborating and working with the lead developer (Daniel) over the years.”
Dimitris and Oliver (Bayer):
“Implementation of a dose escalation study can be achieved without requiring in-depth knowledge of R programming. Comprehensive supporting documentation, available vignettes and well structured code base enables easy implementation of new design features. Collaboration with the
crmPackdevelopment team has been rewarding over the past few years.”
Uli (Roche):
“We started to use CRM dose escalation designs in early development oncology and non-oncology more than 10 years ago. At the beginning
crmPackwas not yet available and especially the simulation part was a little cumbersome. In the meantime,crmPackis our standard package used for CRM dose-escalation methods. Based oncrmPackwe also developed templates for the protocol sections and appendix, which includes simulations for different assumed dose-toxicity relationships. With the help ofcrmPacka model based dose escalation design is no longer a burden also for new-comers and colleagues who have not worked in early development before.”
Anonymous (CRO):
“Running a CRM trial rather than a 3+3 saved us a year in development time and almost 1 million USD (!) as well as providing greater insight to the relationship between dose and toxicity.”
The current CRAN release 2.0 of crmPack includes an impressive array of features:
knitrThe now active funding model follows the successful rpact approach via a Service Level Agreement with RPACT:
rpactRPACT company and RCONIS joint ventureCurrently, 4 pharmaceutical companies have joined the crmPack funding initiative - and of course there is room for more!
Included services:
The annual flat fee includes the following services:
Optional services:
The crmPack package represents a decade of collaborative development and has proven its value in real-world clinical trial applications. With the collaborative funding model, we can ensure its continued development, maintenance, and growth as the standard tool for adaptive dose escalation trials.
The success of this collaborative model with rpact demonstrates that this approach works effectively for the pharmaceutical industry. By participating in this funding initiative, companies can secure access to cutting-edge dose escalation methodology while contributing to the broader statistical community.
We invite interested pharmaceutical companies and CROs to join this collaborative effort to secure the future of crmPack and advance the field of adaptive dose escalation trial designs - please reach out to if you are interested in a first informal chat!